By Andrea Ruiz
Canada’s health approved Pzifer’s antiviral oral treatment for Covid-19.
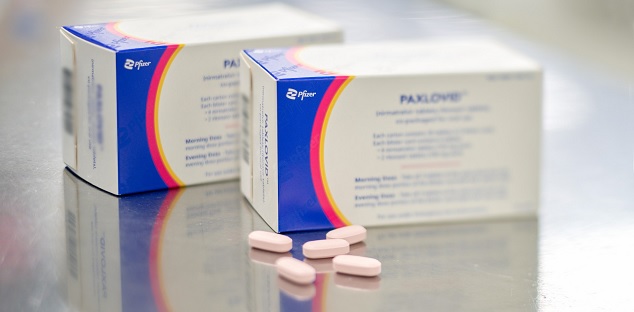
On January 17 Canada’s health approved Pfizer’s COVID-19 oral antiviral treatment.
On their website and press release Pfizer specifies the intentions of the first antiviral of its king the PAXLOVID that “was found to reduce the risk of hospitalization or death by 89% compared to placebo in non-hospitalized high-risk adults with COVID-19”
“Specifically designed to be administered orally so that it can be prescribed at the first sign of infection or at first awareness of an exposure, potentially helping patients avoid severe illness which can lead to hospitalization and death” Pfizer study
This is meant to rewet adults with mild to moderate Covid-19 symptoms and risk of progressive disease, preventing hospitalization and dead. The active ingredient on the paxlovid nirmatrelvir works by stopping the virus from replicating. Statement from Health Canada.
The federal health agency says that the antiviral it is specifically only be given by prescription and for those people that are older than 18. . The treatment consist in a total of six pills taking one daily for no more than five days. Incluiding two pills of nirmatrevlir taken twice a day and one pill of ritonavir taken twice a day. It is important to remark that Plaxlovid cannot be taken if the person is already following treatments for other diseases, including drugs for cancer, high blood pressure and some anti-anxiety and depression medications.To begin the treatment the requirements are that it has to be prescribed by a health expert and must present a positive COVID-19 test.
“no drug, including Paxlovid, is a substitute for vaccination,” said Health Canada’s Chief Medical Adviser Dr. Supriya Sharma, during a technical briefing discussing Paxlovid’s authorization.
This could be an extra tool for Canada’s government to keep people safe and out of the hospitalization risk, but two days after the treatment was approved Prime Minister Justin Trudeau welcomed it on January 19- warning that the Pfizer therapeutic is no substitute for vaccination.
And what are the thoughts and concerns of the people?
“Fantastic news. Isn’t a big issue going to be that it needs to be administered quickly, and with our testing what it is, quickly is all but impossible? “said @HamDanhuis
In response to a tweet from Isaac Bogoch Associate Professor, Department of Medicine, University of Toronto.
People have many concerns as for theantiviral some of them are asking if the amount of antiviral that pfizer will provide to the Canadian goverment will be enough to make an impact and helo to truly fight COVID.
The good news is that Pfizer is now ready to start immediate delivery in Canada, giving priority to patients who are immunocompromised and don’t have enough protection against COVID-19 with vaccines.
Premier Doug Ford said on his Twitter account that “is great news for Ontario providing the health system with another tool” he also said, “We expect to receive approximately 10,000 courses of treatment from the federal government.”
“Canada is going to be getting a 150,000 in treatment courses” According to Kevin Mohamed the Country Lead of Pfizer Canada’s hospital business said for Power Politics this past Monday.


Be the first to comment